Child protection workers found Jo Angel Rodriguez and her siblings living in a roach-infested home that reeked of urine and spoiled food. The kids, often plagued with lice, slept on soiled, squalid mattresses, according to documents filed in a local court. Rodriguez’s mother lost custody of her children in 2006 when complaints surfaced she and her boyfriends shot heroin in front of them. Child protection workers later discovered Rodriguez had been sexually abused by a family member.
The system bounced Rodriguez and her five siblings from one foster home to another over the next two years. But by 2009, things were getting better. The 11-year-old girl finished a stint at a North Texas counseling center, where doctors taught her how to deal with the emotional baggage that comes with abuse and years of neglect. By fall of that year, she rejoined a sister and brother in a San Antonio foster home.
Rodriguez’s foster parents soon complained she was too much to handle, however. In September 2009, they tried unsuccessfully to commit her to Laurel Ridge Treatment Center. Officials with the Texas Department of Family Protective Services were leery, according to one report, suspecting the foster parents had a pattern of dumping kids into psychiatric treatment centers whenever they acted out. Rodriguez had, in fact, already come away from numerous psychiatric evaluations without a clear diagnosis; doctors seemed to agree, though, that she suffered from some mix of anxiety, depression, and post-traumatic stress.
A week later, Rodriguez’s foster parents persisted, claiming she had been aggressive at home and, during time-out one day, said she might harm herself. This time Laurel Ridge jumped into action, admitting the girl and diagnosing her with bipolar disorder.
Staff at the facility gave Rodriguez glowing behavior reports, lauding her for participation in group therapy sessions and for having good manners. One nurse’s report says Rodriguez became protective of the younger girls in her unit.
Two days after her commitment, doctors put Rodriguez on nightly doses of Abilify, an antipsychotic. She began to sleep through the day, becoming withdrawn and refusing to eat. She suffered frequent vomiting and diarrhea.
On September 26, 2009, nurses complained Rodriguez became aggressive when they tried to bathe her.
Dr. Lindy Bankes, a University of Texas Health Science Center resident moonlighting on a weekend shift, prescribed Rodriguez a 1-milligram dose of Risperdal, another antipsychotic. When Rodriguez refused to take the pill, Bankes gave her a 20-milligram shot of Geodon instead.
Three hours later, nurses found Rodriguez in her bed, unable to speak or move, with “shallow and labored” breathing. Due to a stunning breakdown in communication, as evidenced by facility records detailed in court, it took nearly two hours for an ambulance to show. When paramedics arrived, Rodriguez couldn’t move her limbs and had cold, clammy skin. She gasped for air. Her lips were pale, and her blood pressure had tanked so low the paramedic couldn’t get a reading.
Emergency room doctors later determined Rodriguez suffered from a cardiac arrhythmia that triggered a full-blown heart attack. She slipped into a coma and died the next day.
A barrage of errors killed Rodriguez, according to a lawsuit her court-appointed attorneys filed on her behalf in 2010 targeting Laurel Ridge, Bankes, and the ambulance company. They also sued Pfizer, Geodon’s manufacturer, alleging the drug set in motion the deadly chain of events.
Even though the Food and Drug Administration has denied Pfizer’s multiple attempts to approve Geodon for use in children, meaning the company can’t legally market it for pediatric uses, Geodon still made its way into Rodriguez’s room that night.
It wasn’t by accident. The Bexar County lawsuit and several others filed in federal court with the backing of the Texas Attorney General’s Office claim that Pfizer schemed for years to illegally market Geodon to child psychiatrists. In the process, court documents say, Pfizer manipulated information about the drug’s side-effects, paid off doctors to tout the drug’s use in kids, and purchased ghost-written studies that essentially amounted to paid advertising posing as science.
Geodon, a drug known to cause dangerous heartbeat irregularities, is among a new generation of antipsychotics that, while FDA-approved for narrow treatment, have become some of Big Pharma’s signature off-label moneymakers. While the FDA approved Geodon to treat adults diagnosed with schizophrenia and bipolar disorder, Pfizer worked for years to recast the drug for broader use, court records indicate.
Pfizer isn’t alone. The company and its Big Pharma competitors have become the single largest target of the federal False Claims Act, paying out millions in recent settlements over allegations of illegal marketing of drugs, many of them atypical antipsychotics.
“This case is a perfect example, unfortunately, of what can happen” with off-label marketing of pharmaceuticals, contends Brant Mittler, a cardiologist-turned malpractice lawyer who handled the Jo Angel Rodriguez case.
“This child didn’t need to die.”
The 1990s were the Manifest Destiny days for drug manufacturers, and Texas became the first promising frontier. Developed over a half century ago, neuroleptic drugs, now commonly referred to as antipsychotics, were initially seen as little more than powerful sedatives, used sparingly for patients diagnosed with schizophrenia or severe psychotic disorders. Patients often quit the drugs, like Thorazine and Haldol, because they caused involuntary tics, movements, and restlessness.
By the ’90s, however, drug companies began developing new, second-generation antipsychotics, often called atypicals, telling regulators they were safer than the old ones, a dubious claim according to some (non-industry funded) research.
The FDA approved these drugs for treating schizophrenia, but companies dreamed bigger, pushing for the drugs to treat anything from bipolar disorder to dementia, attention-deficit disorder or even stuttering, in all age groups, including children and adolescents.
Texas proved a fertile testing ground. Johnson & Johnson, the first to gain FDA approval for its atypical Risperdal, blazed a trail most of its competitors would soon follow. Through subsidiary Janssen Pharmaceuticals, it trolled for experts hungry for industry cash. Records unearthed in a whistleblower suit that Janssen settled with the State of Texas last year reveal the company paid prominent psychiatrists at Duke, Cornell, and Columbia universities half a million dollars to draft the so-called Schizophrenic Practice Guidelines, draft reports of which were tweaked by Janssen itself.
By 1997, those guidelines became the Texas Medication Algorithm Project, outlining which psychiatric meds doctors should use to treat people in the state’s publicly funded health care system. On the heels of TMAP, the same experts pioneered a spin-off project that touted similar guidelines for prescribing drugs to foster kids.
Court records from the whistleblower suit show drug-industry cash flooded TMAP. All told, Big Pharma subsidized TMAP to the tune of $1.3 million, more than half of which was paid by heavyweights Johnson & Johnson and Pfizer. The TMAP architects — “key opinion leaders” Dr. Steven Shon, then director of the Texas Department of Mental Health and Mental Retardation, Dr. Lynn Crismon of University of Texas’ College of Pharmacy, and Drs. Alexander Miller and John Chiles of San Antonio’s UT Health Science Center — received speaking fees, travel expenses, and honoraria. Shon got more than $30,000, Miller $82,000, and Chiles $151,000 during the TMAP days, according to an expert report filed in Texas’ Medicaid fraud lawsuit against Janssen.
Before the guidelines, the state’s doctors prescribed conventional antipsychotics first, and only reached for the more expensive atypicals after two or three conventionals failed. Under TMAP, atypicals became first-choice treatment options, while conventionals moved down the ladder. In 2002, Texas experts added Geodon as a first-choice medication.
At least 16 states eventually incorporated these guidelines until 2004, when Allen Jones, an investigator with Pennsylvania’s Office of Inspector General, blew the whistle, calling TMAP “a Trojan horse embedded with the pharmaceutical industry’s newest and most expensive mental health drugs.”
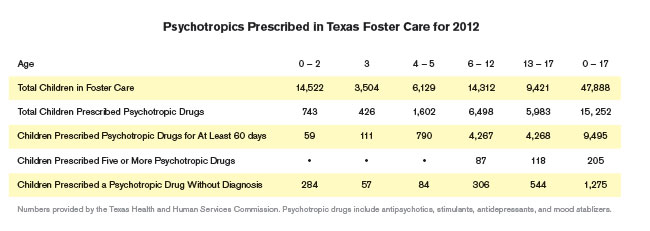
The allegations triggered an investigation by the Texas Comptroller’s Office, which delivered a scathing report on overuse of antipsychotics in state foster care. The Comptroller highlighted nightmare cases like the three-year-old foster child taken to the emergency room for psychotropic poisoning, or that of the six-year-old kid given 60 prescriptions, including mood stabilizers and antipsychotics.
By 2004, Texas foster kids got 23,812 prescriptions for Risperdal, which had yet to be approved by the FDA for use in children, according to the Comptroller’s report. Doctors wrote foster kids some 3,330 prescriptions for Geodon that same year.
In 2005, in reaction to the whistleblower lawsuit, Texas drafted the Psychotropic Medication Utilization Parameters for Foster Children, which were last updated in late 2010, authored by some of the same industry-friendly doctors outed by the Texas AG’s lawsuit. The current parameters cite a 2007 article that appeared in the European Journal of Child and Adolescent Psychiatry boosting the pediatric profile for atypicals, ghost-written by Excerpta Medica and overseen by Janssen, according to an investigation report filed in the state’s lawsuit. The report cites a litany of emails that show Janssen helped craft the article.
Dr. Howard Brody, who heads the Institute for Medical Humanities at the University of Texas Medical Branch, asserts such ghost-writing has “poisoned the medical literature” in recent years, particularly when it comes to atypical antipsychotics.
According to Brody, federal and state governments have sued Pfizer more than a dozen times over the past decade, resulting in millions of dollars in settlement payouts to quell allegations of illegal marketing of drugs, including Geodon.
Pfizer declined to comment on the allegations contained in any of the lawsuits against the company, saying through a spokesperson, “Pfizer remains committed to conducting its business with the highest degree of ethics and integrity, and providing patients, physicians, and the public with accurate, science-based information regarding our medicines.”
“Each time, they swear on a stack of Bibles, ‘Oh we’ll never do that again,’ and they just do it over and over again,” Brody said. “Industry treats this as nothing more than a cost of doing business.”
The FDA rejected Geodon in 1998, just five months after Jo Angel Rodriguez was born, fearing the drug’s tendency to cause cardiac arrhythmia was too great. While the effect is a common one in antipsychotics — known as QT prolongation — the FDA’s disapproval letter to Pfizer expressed concern that Geodon’s impact was worse than that of other antipsychotics on the market.
Pfizer quickly started more clinical trials. The manufacturer wanted to ensure good results, the feds have alleged, so, along with comparing the drug to other leading atypicals, Pfizer put Geodon up against Thioridazine, a highly potent antipsychotic that’s rarely used because of a strong risk of cardiac complications; the FDA had, in fact, already made Thioridazine a second-line treatment because of those concerns.
Some of the data from Pfizer’s clinical trials came from unsavory characters, according to federal court records. Early participants included Drs. Richard Borison and Bruce Diamons, former psychiatrists with the Medical College of Georgia who were later convicted and sentenced to prison for stealing more than $10 million in clinical research funds from their school. Another trial participant was Louis Fabre, founder of Houston’s Fabre Research Clinic, which the FDA blamed for a patient death during a 2002 clinical trial on another antipsychotic.
Over strong objections from staffers, in 2001 the FDA approved Geodon for treating adults with schizophrenia and bipolar disorder. But federal whistleblower lawsuits, in which Texas has been a plaintiff, filed before and after Jo Angel Rodriguez’s death allege that in order to boost Geodon sales, Pfizer followed Johnson & Johnson’s lead, launching a full-court press to market Geodon for off-label use, particularly in children and adolescents.
At least twice the FDA chided Pfizer for providing doctors misleading information. One September 2002 warning letter scolded the company for misrepresenting clinical and safety studies related to the drug, and for promoting Geodon “in a manner that is misleading and lacking fair balance” by minimizing the risk of cardiac arrhythmia. The FDA again warned Pfizer in 2007 for similar problems.
Meanwhile, Pfizer sought to seed the medical literature with studies praising off-label use of Geodon, some of which the federal government described as little more than a marketing gimmick in their lawsuit.
According to another whistleblower suit, Pfizer began to contract with Dr. Neil Kaye, a prominent psychiatrist with Jefferson Medical College in Philadelphia, to promote off-label prescribing of Geodon in medical journals, at speaking gigs, and in continuing medical education seminars for psychiatrists.
Pfizer, according to court records, paid Kaye as much as $4,000 a day on top of expenses, and even flew him around the country in his own private helicopter.
In one court case, the feds called the practice “information laundering.” Apparently it paid off. According to court records, doctors wrote 89,000 Geodon prescriptions for children in the U.S. in 2003. By 2005, prescriptions for children jumped to 251,000.
Three weeks before Jo Angel Rodriguez died at Laurel Ridge, Pfizer paid $2.3 billion to settle claims that it fraudulently marketed Geodon and two other medications. It was the Justice Department’s largest ever health care fraud settlement, and as part of the deal Pfizer agreed to comply with a more rigorous corporate compliance regimen.
But according to another whistleblower’s suit filed a year after Rodriguez’s death, the illegal marketing never stopped.
In 2009, Pfizer again sought approval to market Geodon for use in children; the FDA rejected the application late that year. Further, the FDA issued a report after looking into Pfizer’s data on clinical trials in kids and adolescents, detailing some 24 cases where children experienced serious complications, including cardiac arrhythmia. While Pfizer told the FDA cardiac concerns were no different in children than in adults, the FDA did its own analysis that showed otherwise. The FDA in 2010 scolded three doctors who participated in Geodon’s children trials, saying they improperly dosed patients, and in a few instances overdosed patients.
Emails from executives in Pfizer’s Missouri-based southern district, which also covers Texas, Oklahoma, Colorado, and Louisiana, warned sales teams to shy away from the issue while pitching the drug to child psychiatrists. “[I]nformation related to Geodon’s pediatric filing status should NOT be discussed,” read one such exchange.
Meanwhile, Pfizer continued to pay doctors to market Geodon beyond its FDA-approved uses.
Alex Booker, a sales representative out of Pfizer’s Missouri office, blew the whistle after he was fired for complaining about the pervasive off-label marketing. In court documents, he claimed Pfizer told sales teams to target child psychiatrists with a high number of Medicaid patients — one document filed in court shows Pfizer’s “Medicaid Pull-Through” strategy, listing some two dozen child psychiatrists in the St. Louis area Pfizer hoped would someday write Geodon prescriptions.
Other court filings show that sales reps encouraged Texas Medicaid doctors to boost Geodon doses to up to 360 milligrams per day; the drug was only approved for doses of 160 milligrams, therefore prescriptions over that amount would count as two prescriptions, a quick, easy way for sales reps to exceed quotas and start earning bonuses.
Pfizer, according to the lawsuit, would even go so far as to put undercover sales reps in continuing education seminars. The “plant” would ensure that the group got Pfizer-paid speakers to discuss off-label prescriptions for children.
Booker complained to Pfizer’s compliance hotline in October 2009, claiming, among other problems, that Pfizer urged sales reps to manipulate or conceal Geodon’s safety profile. Hearing nothing back, on January 5, 2010, he followed up with an email to corporate compliance.
Pfizer fired him the next day.
Brant Mittler sought similar records in the local lawsuit against Pfizer. By early this year, all defendants had settled except for the drug manufacturer. For months, Pfizer fought the release of those records, including marketing materials and internal communications between regional and local sales staff.
When earlier this year it appeared Bexar County Probate Court Judge Polly Jackson Spencer might force Pfizer to produce, the company started to talk settlement.
Dr. Joseph Hernandez, Jo Angel’s Rodriguez’s admitting doctor at Laurel Ridge, said the use of Geodon became a bright red flag almost immediately after Rodriguez died, according to deposition testimony lawyers filed in court.
Hernandez said he spoke to Dr. Benigno Fernandez, Laurel Ridge’s medical director, wanting to know why the Geodon was given, why he was never called when the girl’s condition worsened, and why it took so long to transfer Rodriguez to the emergency room when she crashed.
When he spoke to Dr. Lindy Bankes, who ordered the drug, “she was obviously upset,” Hernandez recalled. “She was asking me questions about Geodon.”
According to Bankes’ deposition, the Texas Medical Board notified her someone lodged a complaint against her due to the incident — such complaints are confidential, so there’s no telling who filed it. Despite Hernandez’s obvious concerns with the handling of the case, Fernandez and one of Bankes’ trainers from UTHSC wrote letters to the TMB trying to absolve her of any blame, Bankes testified.
Hernandez found it hard to believe Rodriguez would have been aggressive with staff. “That represented a departure” for the girl, he said. Hernandez had spoken with staff over the phone before the girl died, he said. “I was told that she was actually up and active and laughing, which is not consistent with what was documented” in staff notes, he testified. “I’m having a difficult time trying to figure out what happened here … I can’t figure this out from the documentation.”
Bankes, now in private practice in San Angelo, did not respond to calls for comment. Laurel Ridge’s chief operations officer would not comment, except to say, “Laurel Ridge takes the care, safety, and privacy of all clients very seriously.”
In depositions, both Hernandez and Bankes recalled an email Fernandez sent out months before Rodriguez’s death regarding Geodon. It told staff to use Geodon instead of Zyprexa, an atypical antipsychotic that is FDA-approved for treating bipolar disorder and schizophrenia in adolescents, for cost reasons.
“It (Geodon) was on the pediatric unit and readily available,” Bankes testified. “And, in the email that we got from Dr. Fernandez about using Geodon, it didn’t say anything about not on pediatric patients. I mean, if it was there and on the unit and doctors were using it, then I assume it had been approved by Laurel Ridge.”
While Bankes denied that complications from Geodon had anything to do with Rodriguez’s death, Hernandez admitted that the Geodon “led to a cardiac arrhythmia and a subsequent cardiac arrest and the subsequent death.”
Discussing the case last month, Brant Mittler said “nearly every check and balance in the system failed Jo Angel.” The lawsuit blamed the most blatant lapses on Laurel Ridge.
According to a survey of local court filings, no other psychiatric treatment center in town has the history of serious litigation Laurel Ridge has seen in recent years.
On the night of Rodriguez’s death, a nurse’s entry says the girl was ordered to be transferred to the emergency room at 11 p.m. Hospital records show another transfer order a half hour later. At 12:50 a.m., a nurse starting her shift found Rodriguez barely responsive in her bed, struggling to breathe. An ambulance showed up two minutes later and spent 14 minutes on the scene. The ambulance left Laurel Ridge with Rodriguez at 1:06 a.m., arriving at Methodist Stone Oak Hospital, about 3 miles away, at 1:25 a.m.
Jose Rebolledo, a local pediatric cardiologist who submitted an expert report for the plaintiffs in Rodriguez’s case, wrote, “So, according to their own records, paramedics spent 14 minutes on the scene and then took 19 minutes to travel by ambulance at night 3.2 miles.”
Rodriguez’s death occurred during a particularly troubling period for Laurel Ridge. Two years earlier, state investigators cited the facility for personnel shortages that led to patient neglect. On the heels of that, a Dallas Morning News investigation turned up complaints of teenage patients assaulting each other, of repeated mistakes in medication delivery, and squalid conditions. Texas Department of Family Protective Services records from the past two years include an incident in late 2012 when “staff used excessive force while escorting a child into seclusion room which resulted in bruising.” Another incident report stems from when investigators found unauthorized meds in one child’s bedroom. Other deficiencies noted in the past two years include failure to maintain child/caregiver staffing ratios, and lapses in records keeping.
Nurse Loretta Ramos sued Laurel Ridge in 2008, claiming she was fired because she reported numerous violations, including unsafe staffing levels, and “was a witness to the cover-up of facts surrounding [a] patient’s suicide,” concerning the death of a woman placed on suicide watch at the facility. In another instance, in 2010, a male patient on suicide watch stormed out of a group therapy session and was found dead three hours later, having hung himself with a bed sheet. His Laurel Ridge doctor, Allan Lloyd, had his license revoked by the Texas Medical Review Board a year later over unrelated charges.
Months before Jo Angel Rodriguez entered Laurel Ridge, 24-year-old Lauren Green was admitted for mood disorder, bipolar disorder, depression, and suicidal ideation. According to father Tim Green, Lauren became addicted to Demerol at age 19 when doctors prescribed the pain medication to alleviate her cellulitis. In 2009, the family, from the Dallas-Fort Worth area, scoured the country for a treatment center and settled on Laurel Ridge.
Records show that the day Dr. Malathi Koli discharged Lauren from Laurel Ridge; she gave Lauren 13 different prescription medications, including painkillers and antipsychotics. Koli gave Lauren prescriptions for 10 more medications when she left, according to court records. The day after leaving Laurel Ridge, Lauren headed to Austin to stay with an ex-boyfriend, filled the prescriptions and took them that evening. On the phone that night, “she was completely out of it, just a zombie,” Tim said. The ex-boyfriend found her dead the next morning.
The Travis County Medical Examiner wrote that Lauren died from “acute multiple drug toxicity,” explicitly stating that there was no evidence of an overdose. Lauren had taken the medicine as prescribed by Laurel Ridge.
When the family sued, Laurel Ridge settled with the Greens for an undisclosed amount. The malpractice case against Koli went to trial in a Bexar County court last December. The Greens lost, and the court awarded Koli more than $5,000 in court costs, which the Greens now have to pay.
Jason Cruz, Jo Angel Rodriguez’s court appointed attorney and guardian, recalled rushing to the hospital to visit Rodriguez as soon as he heard she’d been taken to the emergency room. “When I saw her, she was basically comatose.”
DFPS conducted its own death review, writing, “It is of concern that it appears that Jo Angel’s physical illness was not being followed closely, nor was any possible reaction to the emergency medication being monitored.” DFPS also criticized the hospital for taking so long to call an ambulance.
Cruz, for his part, is still troubled Rodriguez was committed to Laurel Ridge in the first place
“Bottom line, it was one of those situations where they (the foster parents) were looking for an excuse to get rid of her,” he claimed. “They had tried to get her admitted before. … I spent more time with those kids than arguably any other case I’ve ever had, and that kind of behavior was just not an issue with her.”
The years-long court battle ended with a whimper last week when attorneys met with Judge Spencer to approve a confidential settlement with Pfizer.
“I guess that was the whole point in filing the lawsuit,” Cruz said, “to try to honor Jo Angel and get her siblings some money.”
For Cruz, the outcome is bittersweet. “As attorneys, we feel great that we succeeded,” he said. “But from knowing that little girl, knowing how bright she was, it just feels kind of empty.”